Research
Background to research
Virology and Neurobiology
Virology and molecular neurobiology are distinct fields of study, but they intersect in their investigation of different aspects of viruses and the nervous system. Virology examines viruses' structure, replication, and interactions with host cells, while molecular neurobiology investigates the molecular processes of the nervous system. The overlap arises when studying how viruses invade neurons, alter neuronal function, and cause neurological damage. Both disciplines contribute to understanding the mechanisms of infection and its neurological consequences.
Current research
Dr. Williams' research primarily centres around the pathogenesis and neuropathogenesis of diseases, utilizing in silico, in vitro, and clinical studies. In our research group, we adopt a multidisciplinary approach to delve into the pathogenesis and neuropathogenesis of diseases. Our investigations incorporate a range of methodologies, including clinical studies, in silico analysis, and in vitro experiments. Currently, our research focuses on three key objectives.
Firstly, we aim to explore the impact and function of sequence variations in HIV-1 viral proteins (Subtype C infection) on clinical outcomes in both children and adults living with HIV. Our investigations delve into the underlying mechanisms involving inflammation, metabolism, and cardiovascular risk (Figure 1).
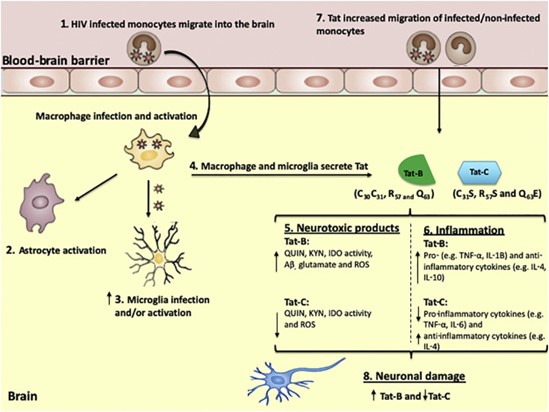
Figure 1: Differential effects of Tat-B and Tat-C in the neuropathophysiology of HIV-associated neurocognitive disorders.
(1) Infected monocytes are able to cross the BBB which later differentiate into macrophages.
(2–3) Infected macrophages within the brain can infect and activate microglia and further activate astrocytes.
(4) These infected macrophages release viral Tat proteins that may directly further activate neuroimmune cells including microglia and astrocytes.
(5) Tat-B induces a higher level of oxidative stress, KP metabolites, Aβ and glutamate which essentially affects neuronal integrity.
(6) Tat- induces a higher inflammatory response compared to Tat C.
(7) Tat-B may be responsible for higher CCL2 levels, greater Blood brain barrier (BBB) damage and essentially higher monocyte transmigration across the BBB. This was largely found to be attributed to the dicysteine motif present in Tat B. (8) In combination, Tat-B may exert its neurotoxic effects to a greater degree than Tat-C and subsequently a greater level of neuronal damage
Secondly, we employ advanced molecular modelling techniques to predict the functional activity of these viral proteins. This approach allows us to gain insights into their structural characteristics and potential effects on disease progression.
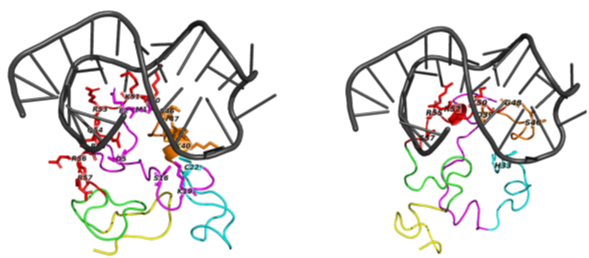
Figure 2 : The top predicted binding poses for TAR docked to Tat subtype B and C. Left Panel shows TAR bound to Tat subtype B and right) TAR bound to Tat Subtype C.
Lastly, we utilize cell culture models to investigate neuroinflammation and neuro-metabolism in various disease conditions. By studying these cellular models, we can better understand the intricacies of disease processes affecting the nervous system.
