In our group we focus on the metabolic changes cancer cells undergo during tumour development and progression. We are particularly interested in two areas. Firstly, the effect of the tumour microenvironment on metastasis and therapy resistance and secondly, the role of one carbon metabolism on cancer cell mitochondrial function
The effect of the tumour microenvironment on metastasis and therapy resistance
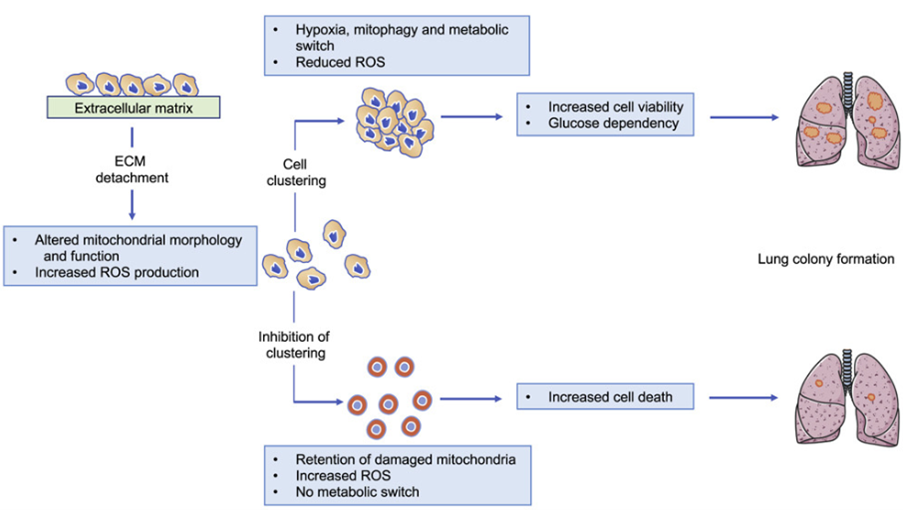
Loss of extracellular matrix attachment
Cancer metastasis depends on cell survival following loss of extracellular matrix attachment and dissemination through the circulation. The metastatic spread can be enhanced by the clustering of detached cancer cells and limiting of reactive oxygen species (ROS) generation. In our research we focus on these processes to find vulnerabilities to target in order prevent metastasis and the spread of cancer.
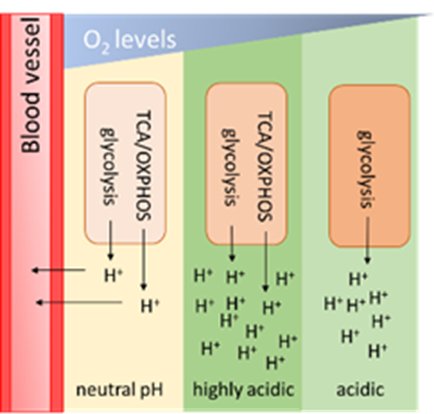
Tumour acidosis
Acidosis, a unique hallmark of tumour tissue is emerging as an important driving force in tumour progression, metastasis, immune evasion, and therapy resistance. Understanding the process of acidification, its impact on tumour invasion and metabolism and how we can exploit it for early detection can greatly improve current treatment strategies.
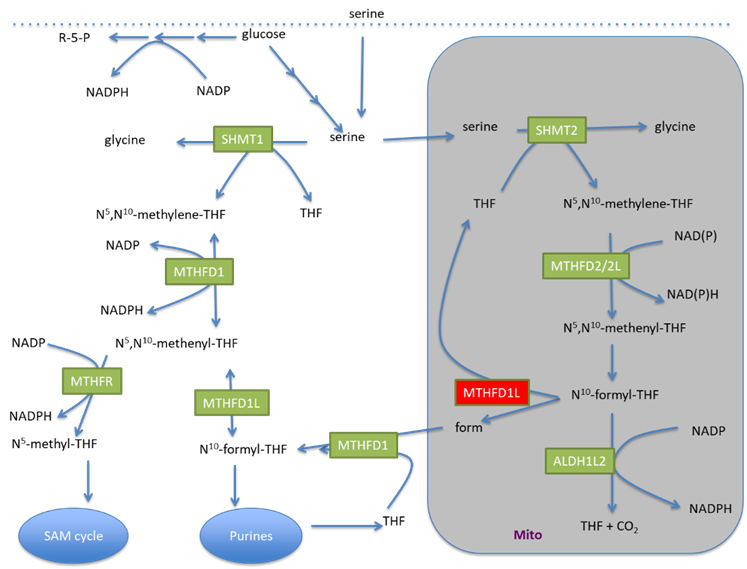
The role of one carbon metabolism in cancer cell mitochondrial function
During cancer development cancer cells undergo metabolic adaptations to satisfy specific metabolic needs during cancer development. Many metabolic pathways have been described to have increased or decreased activity at certain stages of tumour progression that are essential for tumour growth, development and spread. One such pathway is the mitochondrial folate-one carbon cycle. This pathway was shown to be upregulated in different cancers including lung cancer. One carbon metabolism is important to support redox balance, nucleotide synthesis and methylation reactions. One of the enzymes in this pathway is MTHFD1L that converts 10-formyl-THF and ADP into formate, THF and ATP that can be used by other pathways. The expression of this enzyme is increased in lung cancer tissue compared to normal tissue. Interestingly the flux through this enzyme exceeds the need of formate for nucleotide synthesis as formate overflow is observed in cancer cells. Another possible reason for the upregulation of this pathway is to support redox homeostasis and mitochondrial function.
The effect of the tumour microenvironment on metastasis and therapy resistance
Mitochondrial morphology and function in lung cancer cells: an in vitro model system for metastasis.
During metastasis cancer cells need to detach from the primary tumour, and for cells to survive in detached conditions, certain adaptations need to be made including mitochondrial function and morphology. However, it is not clear if these adaptations remain after reattachment. In this project, we will observe the changes in mitochondrial function and morphology in detached cells and determine if these changes remain after reattachment.
Investigating the metabolic changes lung cancer cells undergo during tumour acidosis.
Tumour acidosis have been evidenced to play an important role in cancer progression by aiding metastasis and drug resistance. Tumour acidosis is a key feature within the TME which have metabolic alterations as consequences. It is not well characterised how tumour acidosis affects lung cancer cells’ metabolism, proliferation and migration. The metabolic effects of tumour acidosis have not been fully characterized which is essential for intervening options from a metabolic perspective to provide a better understanding and further insights for potential therapeutic opportunities. It is also essential to consider the heterogeneity among the TME and explore the differences in metabolism at different acidic pH levels. There have been studies investigating metabolic alterations due to tumour acidosis, but most of these studies focused on singular pathways within different types of cancer cell lines (including breast cancer, colon cancer, pharynx cancer and cervix cancer) without considering the full extent on the cancer cells’ metabolism. Lung cancer specifically lacks metabolic studies with no literature available regarding the effects of tumour acidosis on the cells’ metabolism, which is of particular relevance considering that different cancers exhibit differential metabolic adaptions within the tumour microenvironment.
The role of one carbon metabolism in cancer cell mitochondrial function
The role of MTHFD1L in lung cancer cell proliferation, migration, & mitochondrial function.
Several online databases show that MTHFD1L expression is also upregulated in lung cancer tissue, and patients with high MTHFD1L have a lower survival rate. However, no studies have investigated the effect of MTHFD1L silencing on cell proliferation and migration in lung cancer, or the effect on mitochondrial function and morphology.
In this study we will evaluate MTHFD1L as a therapeutic target to inhibit cancer growth and metastasis. This research can ultimately lead to the development of improved and novel treatments for lung cancer.
