In an effort to create more sustainable outcomes for the mining and energy industries, the Hydrometallurgy research group is teaming up with the NWU Minerals Beneficiation research group at the Faculty of Engineering's Centre of Excellence for Carbon based Fuels (CoE for CbF) to conduct joint research as a cluster. This cluster is well-equipped to explore both fundamental and applied areas related to mineral beneficiation and energy storage solutions. They have expertise in a range of topics, including atmospheric and pressure leaching, separation technologies such as precipitation, solvent extraction, pertraction, ion exchange, crystallization, and cementation. This team is also proficient in applied membrane technologies, with experience in electrolyte development and performance optimisation and testing of various redox flow batteries (RFBs).
The objective is to develop innovative solutions capable of transforming the mining and energy sectors. This goal is currently being pursued through the exploration of the following topics:
- Novell separations technologies
Pertraction research undertaken at NWU, CRB since 2011 has been centred around the advancement of a pertraction refinery as an alternative separation technique to conventional solvent extraction. In addition to the optimisation of mass-transfer for specific applications, the research has also focused on the improvement of equipment used in this refining technique.

Cobalt/Nickel Separation
The separation of cobalt and nickel is challenging due to these species’ similar physiochemical properties. Although pyrometallurgy is the most common technique in the extractive metallurgy industry, hydrometallurgy is becoming more popular due to its lower carbon emissions. Solvent extraction is the most effective method for separating and recovering cobalt and nickel from sulphate solutions, and the technique is widely used in the industry because of its high separation efficiency. The technique involves contacting an organic and aqueous phase, with the former containing the extractant and the latter containing the metal of interest. Research has and is currently focusing on optimizing the use of static mixing elements and hollow fibre membrane contactors as an alternative technology to the more traditional mixer-settler configuration. This technique involves using gas-liquid contactors in typical liquid-liquid pertraction operations to allow the organic extract containing the extracted metal to pass through the microporous separator. Hybrid pertraction (HPX) for Co/Ni separation, a new technique developed at the NWU, CRB, allows phase separation to occur in a hydrophobic membrane, reducing liquid holdup and improving efficiency. This method is expected to be 2-3 times quicker than conventional pertraction and the HPX technique is currenly being scaled up to a demonstration plant at the NWU.
YouTube Link
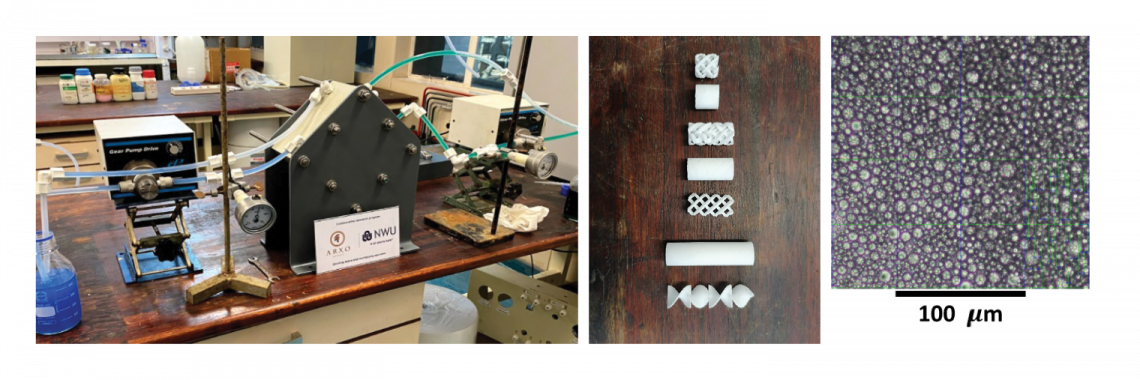
PGM (platinum group metals) are essential elements in various industrial applications, including automobile catalysts, electronics, jewellery, and medical equipment. As the demand for these metals continues to grow, it becomes increasingly important to develop efficient and cost-effective methods for their separation and recovery. PGM ores are typically complex, containing several different metals, making separation challenging. The focus of this project is to selectively extract platinum, palladium, and rhodium from a South African base-metal sulphide matte. In this research the aim is to optimise a selective two step leaching process for the production of a PGM-rich pregnant leach solution. From this, the extraction, scrubbing and stripping isotherms for the PGM target metals are determined through shake-out tests and this liquid-liquid equilibrium data is used for the optimisation of the PGM selective recovery through pertraction technology. After mass-transfer optimisation, a PX-stack module is designed and constructed for laboratory testing and to optimise the operating conditions as a cost effective alternative technique for the selective recovery of PGMs.

- Renewable Energy Storage
Lithium-ion batteries (LiBs)
LiBs are important for various devices, and research in their production is critical for better performance, cost reduction, and sustainability. A mining operation collaborating with this group anticipates producing a multi kiloton heap sulphate-rich pregnant leach solution that contains sufficient lithium to produce several tons of Li per month. It is required that lithium in the PLS be selectively extracted to increase its concentration to an economically feasible amount. The concentrate also contains Na, which must be separated to produce high-purity lithium carbonate or lithium hydroxide. Further purification is also possible to obtain high-purity lithium carbonate used in the manufacturing of lithium-ion battery cathodes and high-purity lithium salts. Currently a study is being conducted to assess the feasibility of solvent extraction separation and recovery of Li from a sodium rich alkaline solution while another study at focusses on the development of LiOH-CO2 absorption and the Li2CO3 crystallization/hot filtration technology to be operated simultaneously. In another study a laboratory counter-current decantation (CCD) leaching system are designed and tested for the feasibility of simultaneous leaching and solid-liquid separation of this metal oxide slag.

Iron-chromium redox flow batteries (ICRFBs)
ICRFBs have emerged as a promising energy storage technology for large-scale applications. South Africa is among the top two countries globally with the highest chromite ore reserves, which makes the production of ICRFB electrolyte from ferrochrome alloy a lucrative option. To achieve this, the FeCr alloy needs to be leached into a liquid phase and impurities removed, which can be achieved through hydrometallurgical processes such as leaching and cementation. In-situ leaching, heap leaching, tank leaching, and fluidized bed reactors (FBRs) can be used for leaching, with FBRs being the most suitable for continuous production processes due to their automation and better control of operating parameters. A small-scale (10 L/h) continuous plant for ICRFB electrolyte production using an FBR was therefore subsequently designed and constructed using the optimised leached conditions developed in another study.
The efficient operation of ICRFBs relies on the maintenance of a balanced electrolyte solution. Rebalancing of the electrolyte solution is necessary to prevent the accumulation of metal ions, which can result in decreased battery performance and shortened cycle life. Rebalancing methods for these electrolyte solutions have been developed in this group with the main focus on the development of a hybrid chemical/electrochemical rebalance method using conventional RFB electrolyte species as intermediates, direct reduction of FeCr and the reactor demonstration on prototype level in order to understand the dependencies of imbalance rates. These methods aim to restore the balance of the electrolytes in the battery and maintain a stable state of charge.

- Waste treatment into products
Zero brine
Base metal refineries generate sodium sulphate solutions during the precipitation process, with concentrations of around 100 g Na2SO4/L. Such high-salinity solutions can harm nearby water sources, so treatment is necessary. In this cluster a cost-effective process using continuous electrodialysis with bipolar membranes and disc reverse osmosis to create potable water, sodium hydroxide, and sulphuric acid was developed. Initially, the process was performed on a small batch scale and more recently, a continuous pilot plant with a capacity of 1.5 kg Na2SO4/h (3 kWDC) combined with a UHP-DRO (Ultra High Pressure – Disk Reverse Osmosis) unit to produce caustic (60 g NaOH/L), acid (40 g H2SO4/L) and process water
(< 1 g Na2SO4/L) was commissioned. The project is currently prepared to be performed on industrial scale.

Selective recovery of cobalt and aluminium from spent catalyst
The Fischer-Tropsch catalyst of choice consists of cobalt, platinum, and aluminum. Recently, the group has initiated efforts to recover the cobalt and aluminum from these spent catalyst. Solvent extraction techniques were utilized to optimize the separation of the cobalt and aluminium, which would be used for agricultural purposes. Shake-out tests were conducted to determine optimal separation conditions and to calculate mass-transfer coefficients. Experimental results were validated by OLI sensitivity studies and finally, a conceptual pertraction refinery was designed to achieve the desired recovery and separation. This project is now ready to be tested on industrial scale at a local beneficiation company.
Rare earth recpovery from coal fly ash
Rare earth elements (REEs), which include the fifteen naturally occurring lanthanides as well as yttrium (Y) and scandium (Sc), are relatively more abundant than precious metals (e.g., gold and platinum). Their unique geochemical properties result in them to be mostly found in ore deposits which are normally not economically feasible to beneficiate. Their unique physiochemical properties (magnetic, electronic, catalytic and optical) do, however, make them crucial feedstocks to the automotive, green technology, electronics and defence manufacturing industries. Recent studies have shown that REEs can be recovered from unconventional resources such as coal fly-ash (CFA) using leaching methods such as alkali fusion-acid leaching and ionic liquid leaching. This is particularly important for developing countries like South Africa, where coal remains an important commodity for energy production. In a more recent study the REE recovery from fly-ashes derived from coal-fired power generation using two different leaching techniques are benchmarked.
