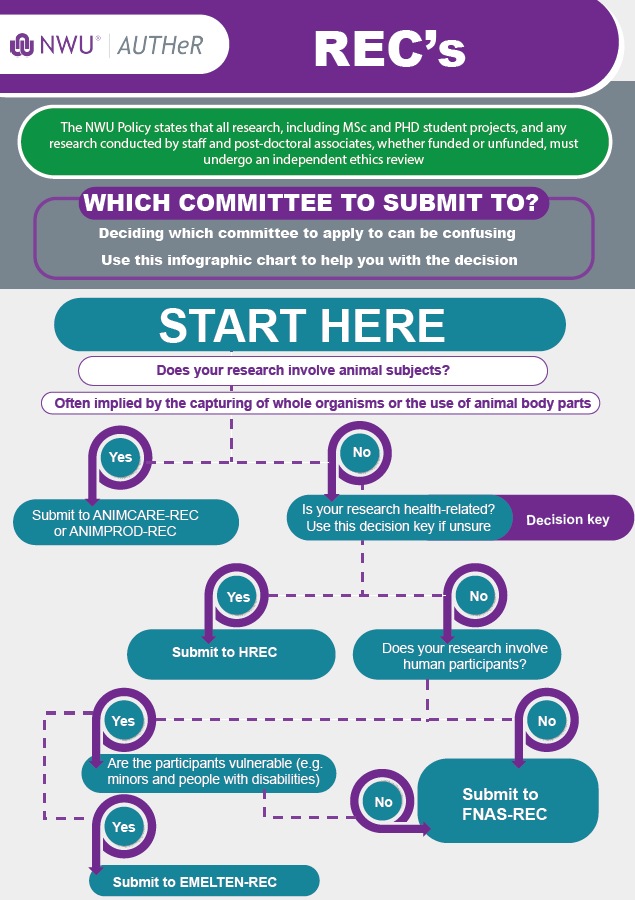
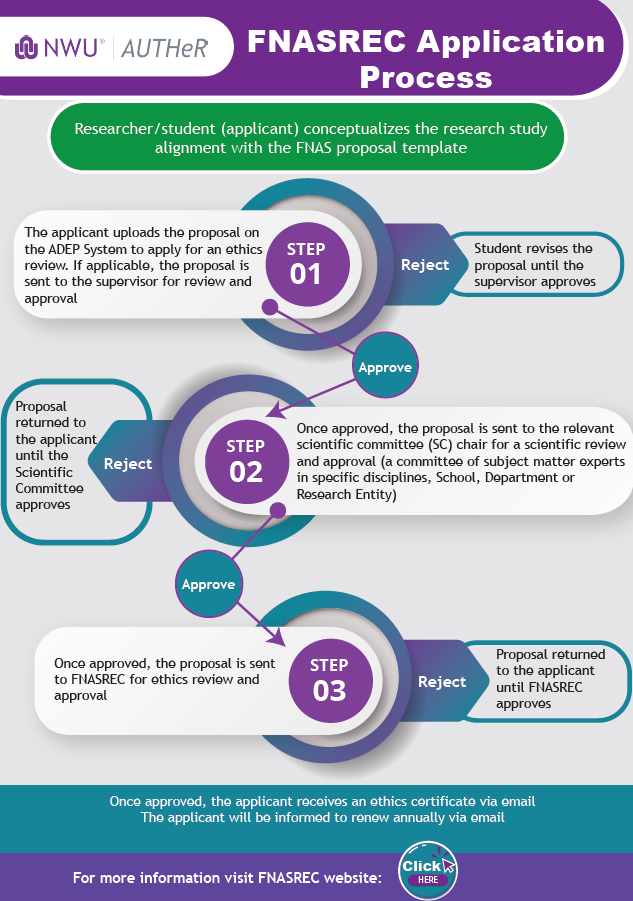
Follow the process below to obtain an ethics number:
-
The student/researcher (applicant) conceptualises the research study in alignment with the ADEP proposal template (this is done in consultation with a supervisor, where applicable)
-
The student/researcher initiates and applies to the ADEP system
-
The proposal is sent to the supervisor for review and approval, if applicable
-
Once approved/submitted, the proposal is sent to the relevant scientific committee chair for review and approval (an internal committee of subject matter experts in a specific discipline, School, or Department)
-
Once approved, the proposal is sent to FNASREC for ethics review and approval.
-
Once approved, the applicant receives an ethics certificate via email.
Here are the essential elements to include in your proposal:
-
Study Purpose and Rationale: Clearly articulate the objectives of your research, its significance, and how it contributes to the broader field of knowledge. Explain why this research is important and justify the need for the study.
-
Population Description: If you use human subjects in your research, describe the target population for your study, including any specific inclusion or exclusion criteria. Highlight the characteristics that make this population relevant to your research objectives.
-
Sampling Strategy: If you use human subjects, detail your approach to selecting participants, including the type of sampling method (e.g., random, stratified, purposive) and the rationale behind this choice. Explain how this strategy will help achieve a representative sample of the population. The ethics committee might ask the question if the potential benefits from the study are fairly distributed, for example, do you give everyone a fair chance to participate in the study.
-
Negotiating Access: If you need access to a community or property, outline the steps you will take to gain access to the population or data. This includes any permissions required from institutions, communities, or individuals and how you plan to address potential access challenges.
-
Informed Consent: Describe the process for obtaining informed consent from participants, ensuring they understand the purpose, procedures, risks, benefits, and their rights within the study. Include how consent will be documented and the measures taken to ensure comprehension for participants with varying levels of literacy or language proficiency.
-
Confidentiality and Anonymity: Detail the measures you will implement to protect participants' confidentiality and anonymity. Explain how data will be stored, who will have access to it, and how long it will be retained before being securely destroyed.
-
Risk Assessment and Mitigation: Assess any potential risks to participants or the environment arising from the research and how these risks will be minimized. This includes physical, psychological, social, or legal risks. The risks can also be to the researchers themselves, like safety. Reputational risk to the NWU should also be considered. If the topic is sensitive, this will change the risk level of the study.
-
Benefits of the Research: If you have a low-risk study, it is important to discuss the potential benefits of your research, both to the participants and the wider community or field of study. Highlight how the study aims to contribute positively to the area of research.
-
Data Handling and Analysis: Explain how data will be collected, processed, and analysed, ensuring transparency in your methodologies and respect for participants' data.
-
Withdrawal Procedure: If your study has human participants, outline the process for participants to withdraw from the study, ensuring they understand their right to discontinue participation at any time without penalty.
-
Complaints Procedure: Include a procedure for participants to raise concerns or complaints about the study, ensuring there is a clear path for addressing ethical issues.
-
NWU data or participants: If the study uses data from the NWU, or uses NWU students or staff as research subjects, permission from the NWU gatekeeper will be required. If you will use existing datasets, mention it.
-
Ethical Considerations for Vulnerable Groups: If your study involves vulnerable populations (e.g., children, individuals with disabilities, or marginalized communities), the risk level is higher than minimal, and the study will have to be reviewed by a registered committee.
Addressing these dimensions thoroughly demonstrates to the ethics review committee your commitment to conducting ethical research that respects and protects your participants, which is fundamental to the integrity of the scientific process.
The typical minimum documentation needed to complete an ethics review includes:
-
Research proposal
-
Proof of IRIMS training
-
Signed code of conduct by all researchers involved
-
For low-risk studies, proof of TRREE training or other ethics training
The supporting documents required, differ based on the risk level associated with the study.
Please look over this document if you are unsure of the risk level.